How to Make an Electrolysis Machine V.2
Posted: Sun Nov 11, 2007 4:37 am
Well after a recent interest in an electrolysis machine (located here http://www.spudfiles.com/forums/eloctro ... 11608.html), I decided it was time to rebuild mine (or to be more percise, make a totally new one).
My old one can be found here: http://www.spudfiles.com/forums/how-to- ... 20305.html
I decided this one had to be easy to build, relatively cheap, and everything had to be easily accessible, which it is in all catoagories.
Unlike my first one, I ditched the collection tank and such, as they weren't very neccessary and limited the portableness of the setup. Also looking over the parts list of my old one, there was a lot of excess parts, so this current one was designed with a short parts list in mind. I also wanted a more profesional looking one and not the ghetto/meth lab looking one I first built.
The entire thing cost about 30 bucks, and took a little under 2 hours to assemble. And all the parts came from either Home Depot, or Ace Hardware.
Parts:
Tank
2" coupler
2" threaded adapter
2"-1/2" bushing reducer
3" long piece of 2" pipe sch40
1/2"-1/4" brass reducer
1/4" hex male-male fitting
1/4" ball valve
2- 1/4" hose barb fittings and 2 feet of hose
Electrode set up
2" threaded plug
4- 3" long 1/4" stainless steel bolts
4- 1/4" stainless steel nuts
4- 1/4" stainless steel washers
8- 1/4" neoprene washers
50+ pack of stainless steel lock washers
2- rubber grommets
2- 2" long pieces of 3/16" copper pipe
Other
assorted tools
pvc cement and primer
pipe dope
solder
wire
water and baking soda

Electrode Plug
Step 1
Take a washer and set it on the bottom and position it along the side of the plug where you want the bolt to go through, and mark with a sharpie; like so...

This is done so the you won't drill the hole for the bolt to close to the plug wall, preventing the washer from sitting flush on the bottom.
Step 2
Repeat step 1 three more times, so that you have four dots on the plug bottom. Then drill out the holes with a 1/4" drill bit and scrap off the price tag. I recommend that you then run the drill bit through the hole and apply pressure to the sides with the bit, making the hole larger so that the bolt will easily pass through. You should now have something like this.
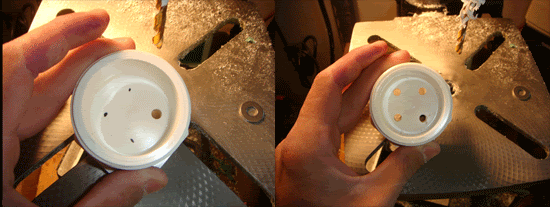
Step 3
Take the pieces of copper pipe, and flatten them. Then position one over the outside holes of the plug, and mark from the inside of the plug with a sharpie. Then drill a hole in the flattened copper with a 1/4" bit, and then widen the holes. Bend an end up slightly, and solder a wire onto it.

Step 4
Put 2 of the bolts throught the holes in the copper, and then put a neoprene washer onto the bolt. Take this and push the two bolts through the holes in the plug.
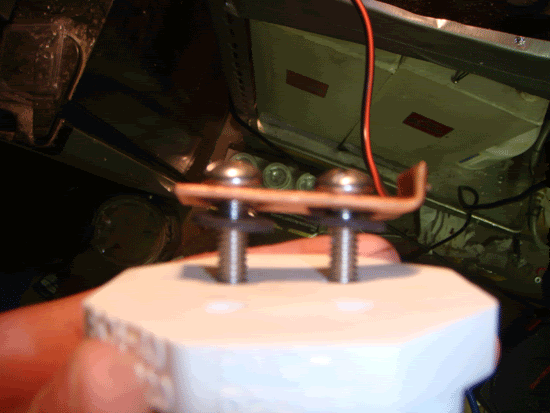
Do this again for the other two bolts, and then put neoprene washers on the bolts protuding out of the bottom of the plug, then a stainless steel washer, and then thread on the nuts and tighten it as best you can.

Step 5.
Finally, add the lock nuts, trying your best to get them not to line up, so that there are gaps between them. Add equal numbers of the washers onto the bolts (or as close to equal as you can get). Then cut the grommets in half and push a hald onto each bolt to keep the washers from falling off when the entire set up is move (Don't push the grommet half as hard as you can, just enough so the washers are loose but don't have a lot of play).

Your done, now onto the electrolysis chamber
Electrolysis Chamber/Water Tank
Step 1
Glue the bushing reducer into the coupler, then the pipe into the other end of the coupler, and then the threaded adapter onto the end of the pipe. Let dry.
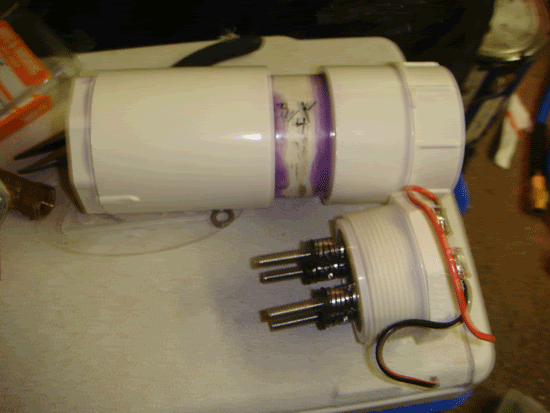
Step 2
After letting dry for 30 mintues or so, go ahead and screw a hose barb into the 1/4" ball valve, then screw the 1/4" ball valve onto the 1/4" hex nipple, then screw that into the 1/2"-1/4" reducer, and then finnaly screw the entire thing into the pvc bushing. Screw a female quick disconnect onto a hose barb and then fit the hose onto both hose barbs, secruing with clamps. (remember to use ptfe/ pipe sealing tape on all threaded connections).

Final Assembly
Take some warm water (about 4 cups) and mix in several table spoons (3 or 4) into the water, continuing to stir until it all disolves and the water is clear.
Take this mixture and pour it into the elelctrolysis chamber (close the ball valve prior to filling). Apply pipe dope to the elelctrode plug, and screw it into the threaded adapter.

Thats it, your done, and the final product....

Notes
This thing has decent gas production, it can fill small balloons with ease, and can pressurize itself to a decent pressure within a few minutes (not sure exact pressure). Currently I use and 8 amp hour lead acid rechargeable battery, but I have plans to upgrade to a larger battery eventually.
Down sides:
1) It's small, so it gets warm after short uses.
2) Because it's small, Gas production isn't amazing, but it is still good for the thing's size.
Upsides:
1) Cheap, easy to get the parts, and easy to build
2) much safer then my old one in that it doesn't leak.
3) Looks pretty good.
Upgrades:
Seeing as I was working on a budget, I went with the washer approach. But most hardware stores sell stainless steel wall outlet plates, and those could be use with a little cutting and bending in this setup to greatly increase surface area.
The water volume could alse be increased to keep the thing from getting so hot rapidly.[/url]
My old one can be found here: http://www.spudfiles.com/forums/how-to- ... 20305.html
I decided this one had to be easy to build, relatively cheap, and everything had to be easily accessible, which it is in all catoagories.
Unlike my first one, I ditched the collection tank and such, as they weren't very neccessary and limited the portableness of the setup. Also looking over the parts list of my old one, there was a lot of excess parts, so this current one was designed with a short parts list in mind. I also wanted a more profesional looking one and not the ghetto/meth lab looking one I first built.
The entire thing cost about 30 bucks, and took a little under 2 hours to assemble. And all the parts came from either Home Depot, or Ace Hardware.
Parts:
Tank
2" coupler
2" threaded adapter
2"-1/2" bushing reducer
3" long piece of 2" pipe sch40
1/2"-1/4" brass reducer
1/4" hex male-male fitting
1/4" ball valve
2- 1/4" hose barb fittings and 2 feet of hose
Electrode set up
2" threaded plug
4- 3" long 1/4" stainless steel bolts
4- 1/4" stainless steel nuts
4- 1/4" stainless steel washers
8- 1/4" neoprene washers
50+ pack of stainless steel lock washers
2- rubber grommets
2- 2" long pieces of 3/16" copper pipe
Other
assorted tools
pvc cement and primer
pipe dope
solder
wire
water and baking soda

Electrode Plug
Step 1
Take a washer and set it on the bottom and position it along the side of the plug where you want the bolt to go through, and mark with a sharpie; like so...

This is done so the you won't drill the hole for the bolt to close to the plug wall, preventing the washer from sitting flush on the bottom.
Step 2
Repeat step 1 three more times, so that you have four dots on the plug bottom. Then drill out the holes with a 1/4" drill bit and scrap off the price tag. I recommend that you then run the drill bit through the hole and apply pressure to the sides with the bit, making the hole larger so that the bolt will easily pass through. You should now have something like this.

Step 3
Take the pieces of copper pipe, and flatten them. Then position one over the outside holes of the plug, and mark from the inside of the plug with a sharpie. Then drill a hole in the flattened copper with a 1/4" bit, and then widen the holes. Bend an end up slightly, and solder a wire onto it.

Step 4
Put 2 of the bolts throught the holes in the copper, and then put a neoprene washer onto the bolt. Take this and push the two bolts through the holes in the plug.

Do this again for the other two bolts, and then put neoprene washers on the bolts protuding out of the bottom of the plug, then a stainless steel washer, and then thread on the nuts and tighten it as best you can.

Step 5.
Finally, add the lock nuts, trying your best to get them not to line up, so that there are gaps between them. Add equal numbers of the washers onto the bolts (or as close to equal as you can get). Then cut the grommets in half and push a hald onto each bolt to keep the washers from falling off when the entire set up is move (Don't push the grommet half as hard as you can, just enough so the washers are loose but don't have a lot of play).

Your done, now onto the electrolysis chamber
Electrolysis Chamber/Water Tank
Step 1
Glue the bushing reducer into the coupler, then the pipe into the other end of the coupler, and then the threaded adapter onto the end of the pipe. Let dry.

Step 2
After letting dry for 30 mintues or so, go ahead and screw a hose barb into the 1/4" ball valve, then screw the 1/4" ball valve onto the 1/4" hex nipple, then screw that into the 1/2"-1/4" reducer, and then finnaly screw the entire thing into the pvc bushing. Screw a female quick disconnect onto a hose barb and then fit the hose onto both hose barbs, secruing with clamps. (remember to use ptfe/ pipe sealing tape on all threaded connections).

Final Assembly
Take some warm water (about 4 cups) and mix in several table spoons (3 or 4) into the water, continuing to stir until it all disolves and the water is clear.
Take this mixture and pour it into the elelctrolysis chamber (close the ball valve prior to filling). Apply pipe dope to the elelctrode plug, and screw it into the threaded adapter.

Thats it, your done, and the final product....

Notes
This thing has decent gas production, it can fill small balloons with ease, and can pressurize itself to a decent pressure within a few minutes (not sure exact pressure). Currently I use and 8 amp hour lead acid rechargeable battery, but I have plans to upgrade to a larger battery eventually.
Down sides:
1) It's small, so it gets warm after short uses.
2) Because it's small, Gas production isn't amazing, but it is still good for the thing's size.
Upsides:
1) Cheap, easy to get the parts, and easy to build
2) much safer then my old one in that it doesn't leak.
3) Looks pretty good.
Upgrades:
Seeing as I was working on a budget, I went with the washer approach. But most hardware stores sell stainless steel wall outlet plates, and those could be use with a little cutting and bending in this setup to greatly increase surface area.
The water volume could alse be increased to keep the thing from getting so hot rapidly.[/url]