Just multiply the volume of your chamber (In Cubic Inches) by the constant 0.008475 This will give you the ounces of CO2 needed at 120psi, 73*f, needed for each chamber fill.
Example:
3.14 x (1/2) x 3"id x 36"length chamber= 254.34ci x 0.008475 = 2.155oz of CO2.
Edit by MrC:
Thanks to Davidvaini and Ragnarok for this calculation table:
http://www.spudfiles.com/forums/viewtop ... tml#229345
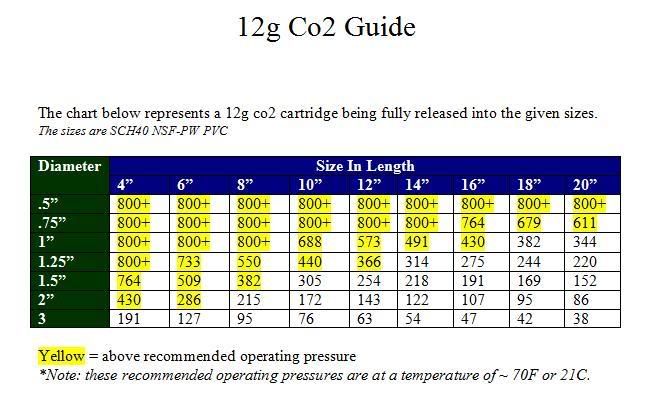
Alternative Word or PDF file available below.







