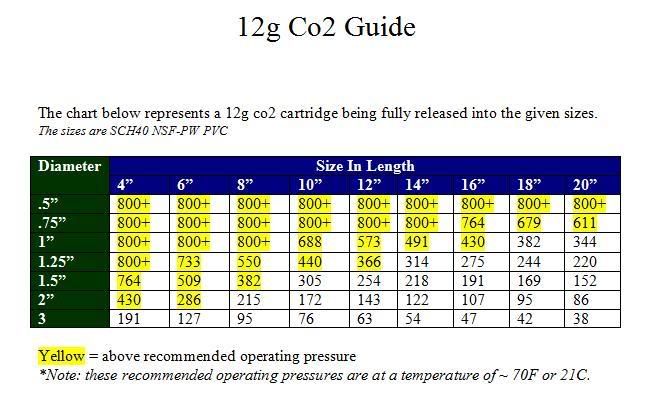
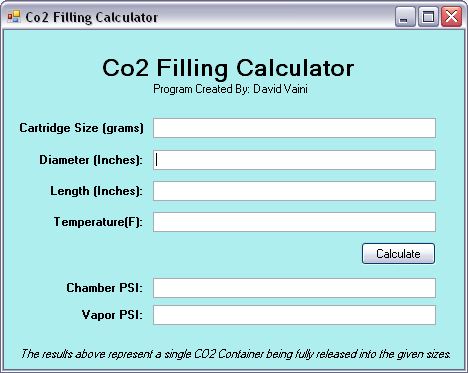
So im guessing he is the one who made one of these laws?BC Pneumatics wrote:Well, most of the credit goes to Clapeyron, but I'm glad I could help.
I am actually surprised to see that i am learning this now in chemistry and it pops up again in the forum.
For anyone who is curious, you can manipulate this formula to solve for different variable but this is the basic equation i leanred.
PV = nRT where:
P = pressure (usually in kPa or atm)
V = volume (usually in Liters)
n = amount of gas (usually in moles)
R = ideal gas constant (8.31 kPa or 0.0821 in atm)
T = Temperature (usually in Kelvin)
So as long as you have all of these variable but one, you cant solve for the missing variable.
And if anyone needs to find the how much pressure, volume or temperature changes depending on what you change, you can use the combined gas formula which is:
P1*V1/T1 = P2*V2/T2 where:
P1 = pressure of first container (usually in kPa)
V1 = volume of first container (usually in Liters)
T1 = temperature of the gas (usually in Kelvin)
P2 = pressure of second container (kPa)
V2 = volume of second container (Liters)
T2 = temperature of second container (Kelvin)
And if one of those variables is missing, then you can solve for it by manipulating the equation.
This was my dollar and 2 cents.